Tuning Maps for Two-Photon Absorption Cross Sections of Canonical and Noncanonical Fluorescent Protein Chromophores
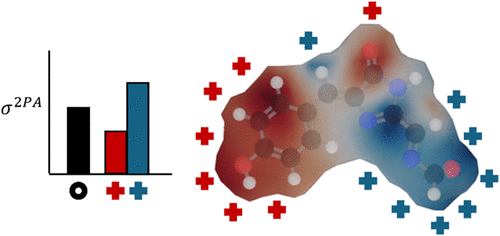
Two-photon absorption (2PA) spectroscopy, which involves the simultaneous absorption of a pair of photons, is an important area of research in chemical biology, where a diversity of biological chromophores have been explored for 2PA applications. This work offers a perspective on how electrostatic interactions influence the 2PA response of fluorescent protein (FP) chromophores, demonstrating the role of their surrounding environment in modulating 2PA optical properties. In this work, we present electrostatic tuning maps, created by placing partial charges along the van der Waals radii of eight different chromophores: Green FP (neutral and anion), Red FP (neutral and anion), mBlueberry FP, Kusabira orange FP, and two noncanonical chromophores, a hydroxyquinolone derivative, and the chromophore of Gold FP. 2PA cross sections (σ2PA) were calculated at the CAM-B3LYP/aug-cc-pVDZ level of theory with and without the presence of point charges. The results show that the influence of point charges varies with their location, leading to substantial increases or decreases in the obtained σ2PA. For the studied systems, neutral chromophores tend to increase their σ2PA when positive point charges are present close to the imidazolinone group, while lowering their σ2PA when they are present near the benzylidene ring. In contrast, anionic species show an opposite trend. Using a positive or negative point charge has the opposite effect as well, increasing or decreasing σ2PA by relatively the same amount. These maps aid in understanding how electrostatic interactions influence 2PA responses, providing a framework for guiding structural modifications to the protein environment as well as the design and optimization of 2PA probes.