Revealing Silica’s pH-Dependent Second Harmonic Generation Response with Overcharging
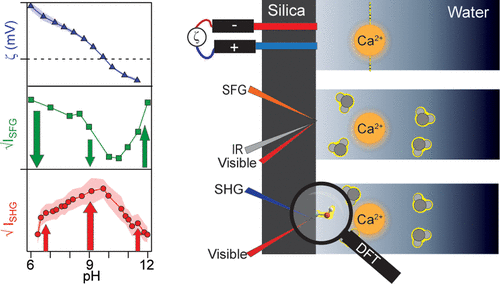
Isolating the contribution of silica in second harmonic generation (SHG) studies at the silica/water interface remains a challenge. Herein, we compare SHG intensities with previously measured zeta potentials and vibrational sum frequency generation (SFG) intensities to deconvolute the silica contribution in the SHG measurements. Under conditions that promote overcharging, the zeta potential and the SFG measurements follow a similar trend; however, SHG yields the opposite behavior. The results can only be rationalized by considering a significant pH-dependent increase in the silica contribution. Using a simplistic, yet physically motivated model, we demonstrate that silica can interfere either constructively or destructively with water. By computing the hyperpolarizabilities of neutral and deprotonated silica clusters with density functional theory [CAM-B3LYP/6-31+G(d,p)], we reveal that one potential source of this pH-dependent response of silica is a change in the hyperpolarizability upon the deprotonation of surface sites, suggesting that SHG is directly sensitive to surface charging. The direct sensitivity of SHG to the surface charge density of the substrate suggests that SHG would be a powerful tool in studying other mineral oxides such as alumina and titania.