Prediction of two-photon absorption enhancement in red fluorescent protein chromophores made from non-canonical amino acids
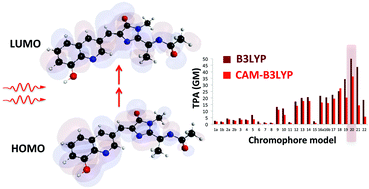
Two-photon spectroscopy of fluorescent proteins is a powerful bio-imaging tool known for deep tissue penetration and little cellular damage. Being less sensitive than the one-photon microscopy alternatives, a protein with a large two-photon absorption (TPA) cross-section is needed. Here, we use time- dependent density functional theory (TD-DFT) at the B3LYP and CAM-B3LYP/6-31+G(d,p) levels of theory to screen twenty-two possible chromophores that can be formed upon replacing the amino-acid Tyr66 that forms the red fluorescent protein (RFP) chromophore with a non-canonical amino acid. The two-level model for TPA was used to assess the properties (i.e., transition dipole moment, permanent dipole moment difference, and the angle between them) leading to the TPA cross-sections determinedvia response theory. Computing TPA cross-sections with B3LYP and CAM-B3LYP yields similar overall trends. Results using both functionals agree that the RFP-derived model of the Gold Fluorescent Protein chromophore (Model 20) has the largest intrinsic TPA cross-section at the optimized geometry. TPA was further computed for selected chromophores following conformational changes: variation of both the dihedral angle of the acylimine moiety and the tilt and twist angles between the rings of the chromophore. The TPA cross-section assumed an oscillatory trend with the rotation of the acylimine dihedral, and the TPA is maximized in the planar conformation for almost all models. Model 21 (a hydroxyquinoline derivative) is shown to be comparable to Model 20 in terms of TPA cross-section. The conformational study on Model 21 shows that the acylimine angle has a much stronger effect on the TPA than its tilt and twist angles. Having an intrinsic TPA ability that is more than 7 times that of the native RFP chromophore, Models 20 and 21 appear to be very promising for future experimental investigation.