Influence of Electron-Donating and Electron-Withdrawing Groups on One- and Two-Photon Absorption Properties on Modified Adenine and Guanine Nucleobases
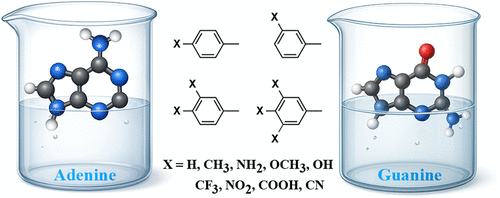
Fluorescent nucleobase analogues are valuable probes for nucleic acid structure and dynamics, yet rational design remains challenging. Here, we investigate one- and two-photon absorption (1PA and 2PA) properties of 66 modified adenine and guanine derivatives using linear response for 1PA and quadratic response theory combined with the two-state model (2SM) for 2PA, employing RI-CC2 and time-dependent density functional theory (CAM-B3LYP and MN15) approaches. Each system includes either electron-donating groups (EDGs: CH3, NH2, OCH3, OH) or electron-withdrawing groups (EWGs: CF3, NO2, COOH, CN) attached at different positions on a phenyl ring appended to the parent adenine or guanine nucleobase. Phenylation enhances the brightness of the parent nucleobases and red-shifts the excitation wavelengths, λ1PA, by up to 47 nm (i.e., 94 nm for λ2PA), while EDG/EWG substitution induces further shifts of up to 113 nm in λ1PA (i.e., 226 nm for λ2PA), thereby extending 2PA activity into the near-infrared region. The largest 2PA responses are associated with substantial permanent dipole changes upon excitation (|Δμ| = [(μ11,x – μ00,x)2 + (μ11,y – μ00,y)2 + (μ11,z – μ00,z)2]1/2 ≈ 13 Debye) and near-colinear alignment between the change in the permanent dipole moment (Δμ) and the transition dipole moment (μ01), cos2θ ≈ 1, as captured by the 2SM. While EDG substitution enhances the photophysical response relative to the parent nucleobases, EWG substitution leads to the most pronounced improvements.