Interplay of Donor-acceptor Interactions in Stabilizing Boron Nitride Compounds: Insights from Theory
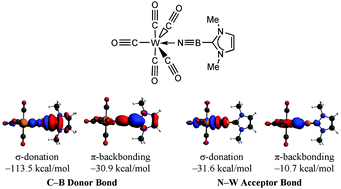
The stability of a variety of linear and cyclic (BN)n (n = 1–3) adducts with N-heterocyclic carbene (ImMe2; ImMe2= [(HCNMe)2C:]), N-heterocyclic olefin (ImMe2CH2) and Wittig (Me3PCH2) donors has been examined using M05-2X/cc-pVTZ computations. The strength and nature of the bonds have been investigated using natural bond orbital (NBO) and atoms-in-molecules (AIM) analyses. Complementary energy decomposition analysis (EDA-NOCV) has been carried out based on BP86/TZ2P computations. In agreement with NBO and AIM analyses, the orbital interaction energy obtained from EDA contributes at least 50% to the total attractive interactions for the carbon–boron bonds indicating their largely covalent nature. The feasibility of isolating monomeric (BN)n units using a donor/acceptor protocol was also investigated in a series of adducts of the general form: LB·(BN)n·BH3 and LB·(BN)n·W(CO)5 (n = 1–3; LB = Lewis bases). Moreover, EDA-NOCV analysis of ImMe2·BN·W(CO)5 and ImMe2·B3N3·W(CO)5 shows that the carbene–boron bonds are stronger in the presence of W(CO)5 as a Lewis acid mainly because of a dramatic decrease in the amount of Pauli repulsion rather than an increase in the electrostatic/orbital attraction terms.