Influence of the Hydrogen Bonding Environment on Vibrational Coupling in the Electrical Double Layer at the Silica/Aqueous Interface,
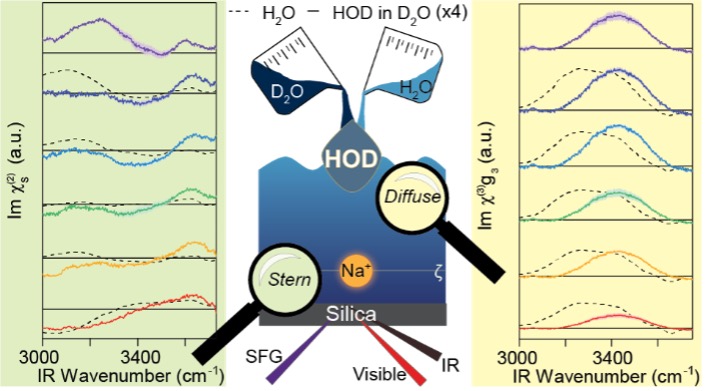
Vibrational spectroscopy is a powerful tool for determining the local hydrogen-bonding environment. However, vibrational coupling present in H2O makes it difficult to relate vibrational spectra to a molecular description of the system. While numerous bulk studies have shed light on this phenomenon, the influence of both intra- and intermolecular vibrational coupling on the resulting electrical double layer spectra at buried interfaces remains largely unexplored. By utilizing vibrational sum frequency generation (vSFG), electrokinetic measurements, and the maximum entropy method on isotopically diluted water (HOD) at the silica/aqueous interface, we reveal the influence of vibrational coupling on the Stern and diffuse layer spectra as the pH is varied. For the Stern layer spectra, we observe differences in the frequency centers at pH 2 that are less significant at higher pH, signifying the presence of intermolecular coupling that can be related to the double-donor hydrogen-bonded structure of water. Furthermore, the differences in the evolution of the Stern layer of H2O and HOD suggest that the presence of intramolecular coupling in the former may distort the spectral response. Moreover, we observe that the evolution of HOD closely matches the pKa of the out-of-plane silanols predicted by previous molecular dynamic simulations.